Co-occurring characteristics of pore gas and water in shales: a case study of the Lower Silurian Longmaxi Formation in the southeastern Sichuan Basin
-
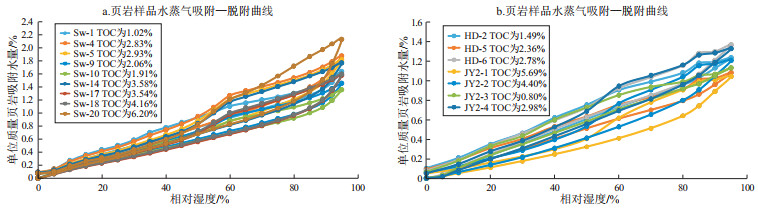
摘要: 以川东南地区下志留统龙马溪组页岩为研究对象,借助重量法水蒸气吸附仪、重量法甲烷等温吸附仪以及页岩组成和孔隙结构等分析手段,开展束缚水、吸附气赋存定量研究,并探讨了微纳米孔隙中气—水赋存特征及主要影响因素。研究表明,不同类型页岩束缚水赋存能力差异较明显,可以利用水蒸气吸附—脱附和GAB模型比较准确地定量描述束缚水特征。页岩最大单层水分子吸附量与黏土矿物含量呈显著的正相关,表明黏土矿物为水分子提供了主要的活性吸附位。页岩对水分子的吸附能力要整体高于甲烷分子,而甲烷分子则主要以单层吸附形式在孔隙中赋存。不同页岩中束缚水、吸附气和游离气赋存的孔隙空间存在差异。2 nm以下孔隙均被吸附气和孔隙水所占据;有机碳(TOC)含量小于2.5%的页岩中游离气主体赋存空间约为5 nm以上孔隙,而TOC含量大于2.5%的页岩中游离气赋存空间主体约为3 nm以上孔隙;有机碳含量越高,游离气赋存的空间占比越高。Abstract: In this paper, the co-occurring characteristics of pore gas and water in the Longmaxi Formation shales in the Sichuan Basin, South China were investigated. Water vapor and methane adsorption by the means of gravimetric methods were carried out to quantitatively determine the behavior of gas and bounding water in micro-nano pores. The impact of the shale compositions and pore structures on the occurring characteristics were discussed. Results showed that the storage capacity of bound water in different types of shales varied dramatically, and the characteristics of bound water could be described by the water vapor adsorption curve and the GAB model. There is an apaprent positive correlation between the maximum monolayer water molecule adsorption capacity and the clay mineral content in shales, indicating that clay minerals provide the main active adsorption sites for water molecules. The adsorption capacity of shale to water molecule is higher than that of methane molecule overall, and methane molecule mainly exist in pores with the form of monolayer adsorption. Bound water, adsorbed gas and free gas could be stored in different pore ranges of different shales. Pores with diameters lower than 2 nm are occupied by bounding water and adsorbed gas. For shales with TOC < 2.5%, free gas would be stored in pores with diameters larger than 5 nm approximately, while for the shales with TOC>2.5%, free gas would be stored in pores with diameters larger than 3 nm approximately. The higher the TOC content, the higher the proportion of the free-gas storage space.
-
表 1 川东南地区下志留统龙马溪组页岩X衍射矿物组成与水蒸气吸附结果
Table 1. X-diffraction mineral composition and water vapor adsorption results of Lower Silurian Longmaxi Formation shale in southeastern Sichuan Basin
样品名称 井号 井深/m ω(TOC)/% X衍射分析结果 单位岩石水蒸气吸附结果 黏土含量/% 石英含量/% 碳酸盐矿物含量/% Wm/% Wm/(mmol·g-1) K C Sw-1 JY11-4 2 271.8 1.02 53.3 40.4 2.0 0.76 0.42 0.60 5.72 Sw-4 2 286.4 2.83 47.5 37.1 6.5 0.70 0.39 0.68 4.83 Sw-5 2 289.8 2.93 40.9 45.8 6.3 0.62 0.34 0.71 4.75 Sw-9 2 308.3 2.06 35.0 51.7 8.5 0.51 0.28 0.7 5.49 Sw-10 2 311.6 1.91 34.1 53.8 5.6 0.49 0.27 0.69 5.73 Sw-14 2 329.5 3.58 35.1 53.9 6.3 0.55 0.31 0.74 5.1 Sw-17 2 342.3 3.54 26.3 58.5 10.6 0.42 0.23 0.78 5.54 Sw-18 2 347.1 4.16 26.1 61.2 6.4 0.43 0.24 0.77 5.81 Sw-20 2 355.9 6.20 25.2 65.9 2.8 0.45 0.25 0.83 6.48 JY2-1 JY2 2 572.5 5.69 18.4 58.3 20.0 0.30 0.17 0.79 2.78 JY2-2 2 566.8 4.40 25.3 63.8 7.4 0.47 0.26 0.71 2.18 JY2-3 2 470.9 0.80 64.3 31.6 1.4 1.83 1.02 0.27 2.41 JY2-4 2 460.3 2.98 44.5 46.3 4.9 0.77 0.43 0.59 2.32 HD-2 PY1 2 130.4 1.49 52.1 35.8 7.3 1.15 0.64 0.42 2.54 HD-5 2 138.1 2.36 41.4 43.0 9.9 0.62 0.34 0.56 3.36 HD-6 2 142.9 2.78 46.4 48.6 2.2 0.64 0.36 0.63 3.95 注:Wm为水分子最大单层吸附量;K为多层吸附势常数;C为单层吸附热常数。 表 2 川东南地区龙马溪组页岩全孔径分布与甲烷等温吸附结果
Table 2. Full diameter distribution and methane isotherm adsorption results of Longmaxi Formation shale in southeastern Sichuan Basin
样品名称 井号 井深/m 全孔径分布结果 甲烷等温吸附结果 比表面积/(m2·g-1) 总孔容/(mL·g-1) VL/(m3·t-1) PL/MPa 甲烷VL/(mmol·g-1) 理论单层吸附量/(m3·t-1) Sw-1 JY11-4 2 271.8 9.8 0.014 25 1.80 3.93 0.08 3.22 Sw-4 2 286.4 18.4 0.024 54 3.14 4.16 0.14 6.04 Sw-5 2 289.8 18.5 0.024 40 3.00 3.66 0.13 6.07 Sw-9 2 308.3 12.4 0.019 86 2.37 4.37 0.11 4.07 Sw-10 2 311.6 11.3 0.018 36 2.32 5.86 0.10 3.71 Sw-14 2 329.5 20.0 0.026 97 3.08 3.66 0.14 6.57 Sw-17 2 342.3 15.4 0.022 95 2.94 3.48 0.13 5.06 Sw-18 2 347.1 19.2 0.026 71 3.24 3.32 0.14 6.30 Sw-20 2 355.9 26.7 0.034 44 4.07 2.76 0.18 8.76 JY2-1 JY2 2 572.5 24.6 0.035 20 3.86 1.16 0.17 8.08 JY2-2 2 566.8 17.3 0.030 00 3.52 1.55 0.16 5.68 JY2-3 2 470.9 9.4 0.007 39 1.68 3.67 0.08 3.09 JY2-4 2 460.3 17.3 0.019 81 - - - 5.58 HD-2 PY1 2 130.4 11.4 0.017 40 2.40 2.57 0.11 3.74 HD-5 2 138.1 11.8 0.023 90 2.14 2.78 0.10 3.87 HD-6 2 142.9 15.6 0.020 60 3.13 2.01 0.14 5.12 表 3 川东南地区龙马溪组不同页岩孔隙中束缚水、吸附气和游离气赋存空间特征
Table 3. Illustration of pores occupied by bound water, adsorbed gas and free gas in different shales in Lower Silurian Longmaxi Formation, southeastern Sichuan Basin

-
[1] SAKHAEE-POUR A, BRYANT S L. Pore structure of shale[J]. Fuel, 2015, 143: 467-475. doi: 10.1016/j.fuel.2014.11.053 [2] AMANN-HILDENBRAND A, GHANIZADEH A, KROOSS B M. Transport properties of unconventional gas systems[J]. Marine and Petroleum Geology, 2012, 31(1): 90-99. doi: 10.1016/j.marpetgeo.2011.11.009 [3] GENSTERBLUM Y, GHANIZADEH A, CUSS R J, et al. Gas transport and storage capacity in shale gas reservoirs-a reviews Part A: Transport processes[J]. Journal of Unconventional Oil and Gas Resources, 2015, 12: 87-122. doi: 10.1016/j.juogr.2015.08.001 [4] 王飞宇, 贺志勇, 孟晓辉, 等. 页岩气赋存形式和初始原地气量(OGIP)预测技术[J]. 天然气地球科学, 2011, 22(3): 501-510. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201103019.htmWANG Feiyu, HE Zhiyong, MENG Xiaohui, et al. Occurrence of shale gas and prediction of original gas in-place (OGIP)[J]. Natural Gas Geosciences, 2011, 22(3): 501-510. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201103019.htm [5] HAO Fang, ZOU Huayao, LU Yongchao. Mechanisms of shale gas storage: implications for shale gas exploration in China[J]. AAPG Bulletin, 2013, 97(8): 1325-1346. doi: 10.1306/02141312091 [6] 方朝合, 黄志龙, 王巧智, 等. 富含气页岩储层超低含水饱和度成因及意义[J]. 天然气地球科学, 2014, 25(3): 471-476. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201403021.htmFANG Chaohe, HUANG Zhilong, WANG Qiaozhi, et al. Cause and significance of the ultra-low water saturation in gas-enriched shale reservoir[J]. Natural Gas Geoscience, 2014, 25(3): 471-476. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201403021.htm [7] 刘洪林, 王红岩. 中国南方海相页岩超低含水饱和度特征及超压核心区选择指标[J]. 天然气工业, 2013, 33(7): 140-144. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201307032.htmLIU Honglin, WANG Hongyan. Ultra-low water saturation characte-ristics and the identification of over-pressured play fairways of marine shales in South China[J]. Natural Gas Industry, 2013, 33(7): 140-144. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201307032.htm [8] HATCH C D, WIESE J S, CRANE C C, et al. Water adsorption on clay minerals as a function of relative humidity: application of BET and freundlich adsorption models[J]. Langmuir, 2012, 28(3): 1790-1803. doi: 10.1021/la2042873 [9] LAHN L, BERTIER P, SEEMANN T, et al. Distribution of sorbed water in the pore network of mudstones assessed from physisorption measurements[J]. Microporous and Mesoporous Materials, 2020, 295: 109902. doi: 10.1016/j.micromeso.2019.109902 [10] SHEN W J, LI X Z, LU X B, et al. Experimental study and isotherm models of water vapor adsorption in shale rocks[J]. Journal of Natural Gas Science and Engineering, 2018, 52: 484-491. doi: 10.1016/j.jngse.2018.02.002 [11] SANG G J, LIU S M, ZHANG R, et al. Nanopore characterization of mine roof shales by SANS, nitrogen adsorption, and mercury intrusion: impact on water adsorption/retention behavior[J]. International Journal of Coal Geology, 2018, 200: 173-185. doi: 10.1016/j.coal.2018.11.009 [12] SANG G J, LIU S M, ELSWORTH D. Water vapor sorption pro-perties of Illinois shales under dynamic water vapor conditions: experimentation and modeling[J]. Water Resources Research, 2019, 55(8): 7212-7228. doi: 10.1029/2019WR024992 [13] BAHADUR J, MELNICHENKO Y B, MASTALERZ M, et al. Hierarchical pore morphology of cretaceous shale: a small-angle neutron scattering and ultrasmall-angle neutron scattering study[J]. Energy & Fuels, 2014, 28(10): 6336-6344. [14] KING H E Jr, EBERLE A, WALTERS C C, et al. Pore architecture and connectivity in gas shale[J]. Energy & Fuels, 2015, 29(3): 1375-1390. [15] CLARKSON C R, SOLANO N, BUSTIN R M, et al. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion[J]. Fuel, 2013, 103: 606-616. doi: 10.1016/j.fuel.2012.06.119 [16] RUPPERT L F, SAKUROVS R, BLACH T P, et al. A USANS/SANS study of the accessibility of pores in the barnett shale to methane and water[J]. Energy & Fuel, 2013, 27(2): 772-779. [17] 李靖, 李相方, 陈掌星, 等. 页岩储层束缚水影响下的气相渗透率模型[J]. 石油科学通报, 2018, 3(2): 167-182. https://www.cnki.com.cn/Article/CJFDTOTAL-SYKE201802005.htmLI Jing, LI Xiangfang, CHEN Zhangxin, et al. Permeability model for gas transport through shale nanopores with irreducible water saturation[J]. Petroleum Science Bulletin, 2018, 3(2): 167-182. https://www.cnki.com.cn/Article/CJFDTOTAL-SYKE201802005.htm [18] SUN Z, LI X F, SHI J T, et al. Apparent permeability model for real gas transport through shale gas reservoirs considering water distribution characteristic[J]. International Journal of Heat and Mass Transfer, 2017, 115: 1008-1019. doi: 10.1016/j.ijheatmasstransfer.2017.07.123 [19] ZHANG T W, ELLIS G S, RUPPEL S C, et al. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems[J]. Organic Geochemistry, 2012, 47: 120-131. doi: 10.1016/j.orggeochem.2012.03.012 [20] GASPARIK M, BERTIER P, GENSTERBLUM Y, et al. Geological controls on the methane storage capacity in organic-rich shales[J]. International Journal of Coal Geology, 2014, 123: 34-51. doi: 10.1016/j.coal.2013.06.010 [21] MERKEL A, FINK R, LITTKE R. The role of pre-adsorbed water on methane sorption capacity of Bossier and Haynesville shales[J]. International Journal of Coal Geology, 2015, 147/148: 1-8. doi: 10.1016/j.coal.2015.06.003 [22] BARRETT E P, JOYNER L G, HALENDA P P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms[J]. Journal of the American Chemical Society, 1951, 73(1): 373-380. doi: 10.1021/ja01145a126 [23] MUSA M A A, YIN C Y, SAVORY R M. Analysis of the textural characterstics and pore size distribution of a commercial zeolite using various adsorption models[J]. Journal of Applied Sciences, 2011, 11(21): 3650-3654. doi: 10.3923/jas.2011.3650.3654 [24] BRUNAUER S, EMMETT P H, TELLER E. Adsorption of gases in multi molecular layers[J]. Journal of the American Chemical Society, 1938, 60(2): 309-319. doi: 10.1021/ja01269a023 [25] 俞凌杰, 范明, 陈红宇, 等. 富有机质页岩高温高压重量法等温吸附实验[J]. 石油学报, 2015, 36(5): 557-563. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201505004.htmYU Lingjie, FAN Ming, CHEN Hongyu, et al. Isothermal adsorption experiment of organic-rich shale under high temperature and pressure using gravimetric method[J]. Acta Petrolei Sinica, 2015, 36(5): 557-563. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201505004.htm [26] TANG X, RIPEPI N, VALENTINE K A, et al. Water vapor sorption on Marcellus shale: measurement, modeling and thermodynamic analysis[J]. Fuel, 2017, 209: 606-614. doi: 10.1016/j.fuel.2017.07.062 [27] SEEMANN T, BERTIER P, KROOSS B M, et al. Water vapour sorption on mudrocks[J]. Geological Society, London, Special Publications, 2017, 454(1): 201-233. doi: 10.1144/SP454.8 [28] ZOLFAGHARI A, DEHGHANPOUR H, XU M X. Water sorption behaviour of gas shales: II. Pore size distribution[J]. International Journal of Coal Geology, 2017, 179: 187-195. doi: 10.1016/j.coal.2017.05.009 [29] DEHGHANPOUR H, ZUBAIR H A, CHHABRA A, et al. Liquid intake of organic shales[J]. Energy & Fuels, 2012, 26(9): 5750-5758. [30] STRIOLO A, GUBBINS K E, GRUSZKIEWICZ M S, et al. Effect of temperature on the adsorption of water in porous carbons[J]. Langmuir, 2005, 21(21): 9457-9467. doi: 10.1021/la051120t [31] MOSHER K, HE H J, LIU Y Y, et al. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems[J]. International Journal of Coal Geology, 2013, 109/110: 36-44. doi: 10.1016/j.coal.2013.01.001 [32] CHEN G H, LU S F, LIU K Y, et al. Critical factors controlling shale gas adsorption mechanisms on different minerals investigated using GCMC simulations[J]. Marine and Petroleum Geology, 2019, 100: 31-42. doi: 10.1016/j.marpetgeo.2018.10.023 [33] 俞凌杰, 范明, 腾格尔, 等. 埋藏条件下页岩气赋存形式研究[J]. 石油实验地质, 2016, 38(4): 438-444. doi: 10.11781/sysydz201604438YU Lingjie, FAN Ming, TENGER, et al. Shale gas occurrence under burial conditions[J]. Petroleum Geology & Experiment, 2016, 38(4): 438-444. doi: 10.11781/sysydz201604438 [34] REXER T F, BENHAM M J, APLIN A C, et al. Methane adsorption on shale under simulated geological temperature and pressure conditions[J]. Energy & Fuels, 2013, 27(6): 3099-3109. -





 下载:
下载:




 苏公网安备32021102000780号
苏公网安备32021102000780号