Screening of functional monomers and preparation of molecularly imprinted polymers (MIPs) in molecularly imprinted polymers of steranes
-
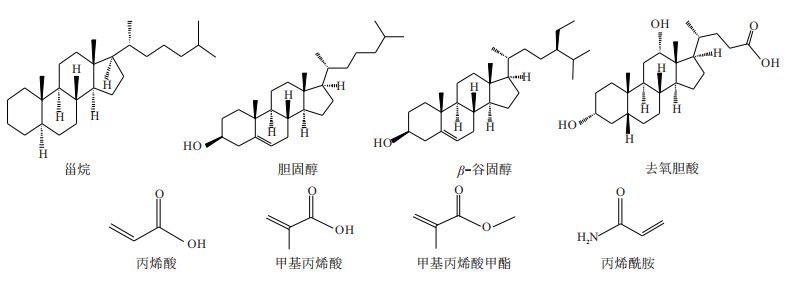
摘要: 为了制备对甾烷类化合物具有特异性选择能力的分子印迹聚合物(MIPs),利用紫外光谱法对预聚合体系进行筛选,确定功能单体的种类、配比及其作用方式。将3种模板分子(胆固醇、β-谷固醇、去氧胆酸)分别与4种功能单体,即丙烯酸(AA)、甲基丙烯酸(MAA)、甲基丙烯酸甲酯(MMA)、丙烯酰胺(AM))的相互作用强度进行对比。研究结果发现,功能单体AA均可与3种模板分子发生较强的相互作用并形成稳定的预聚合体系,从而优选出AA为MIPs的功能单体。另外,通过不同比例AA紫外光谱吸光度变化和差示紫外光谱分析,显示3种模板分子与功能单体AA的最佳浓度比均为1∶4,且形成的稳定配合物构型分别为胆固醇-1AA、β-谷固醇-1AA及去氧胆酸-3AA。同时,以EDGMA为交联剂、AIBN为引发剂,采用沉淀聚合法成功合成了MIPs,傅立叶变换红外光谱(FTIR)分析结果显示MIPs制备良好。因此,此方法可用于对甾烷类化合物具有特异性选择能力的MIPs功能单体的筛选及其制备,并为深层—超深层烃源岩油源对比研究提供技术支持。Abstract: To prepare molecularly imprinted polymers (MIPs) with specific selectivity for steranes, the prepolyme-rization system was screened by UV spectroscopy to determine the type, proportion and mode of action of functional monomers. In this study, the interaction intensities between three template molecules (cholesterol, β-sitosterol, deoxycholic acid) and four functional monomers (acrylic acid (AA), methacrylic acid (MAA), methyl methacrylate (MMA), and acrylamide (AM)) are compared respectively. The results show that the functional monomer AA can interact strongly with the three template molecules and form a stable prepolymerization system, so AA is selected as the functional monomer of MIPs. In addition, the UV spectral absorbance change and differential UV spectral analysis of different proportions of AA show that the optimal concentration ratios of the three template molecules to functional monomer AA are all 1:4, and the stable complex configurations formed are cholesterol-1AA, β-sitosterol-1AA and deoxycholic acid-3AA, respectively. The MIPs are successfully synthesized by precipitation polymerization using EDGMA as dispersant and AIBN as initiator, and the results of FTIR show that MIPs are well prepared. Therefore, this method can be used for the screening and preparation of MIPs functional monomers with specific selectivity for steranes, and provide technical support for the study of oil-source correlation between deep and ultra-deep source rocks.
-
Key words:
- steranes /
- molecularly imprinted polymer /
- UV spectrum /
- template molecule /
- functional monomer
-
表 1 制备分子印迹聚合物(MIPs)相关实验的主要试剂
Table 1. Main reagents used in related experiments for preparing MIPs
试剂名称 试剂级别 生产厂家 胆固醇 AR 天津市大茂化学试剂厂 β-谷固醇 AR 合肥巴斯夫生物科技有限公司 去氧胆酸 AR 麦克林生物科技有限公司 AA AR 天津市大茂化学试剂厂 MAA AR 天津市大茂化学试剂厂 MMA AR 天津市大茂化学试剂厂 AM AR 上海广诺化学科技有限公司 EDGMA AR 北京百灵威科技有限公司 AIBN AR 天津市大茂化学试剂厂 甲醇 HPLC 北京迈瑞达科技有限公司 乙腈 HPLC 北京迈瑞达科技有限公司 表 2 制备MIPs相关实验的主要仪器设备
Table 2. Main instruments and equipments used in related experiments for preparing MIPs
实验仪器 型号 生产厂家 紫外可见光度计 UV-2600 谱质分析检测技术(上海)有限公司 傅立叶红外光谱仪 Bruker Alpha 泰科施普(北京)技术有限公司 电子恒温不锈钢水浴锅 HHS-2S 上海虞龙仪器设备有限公司 场发射扫描电子显微镜 Merlin Compact 德国蔡司 气相色谱/质谱联用仪 6890N(GC)/5737N(MS) 美国安捷伦科技有限公司 回旋式振荡器 HY-5 江苏盛兰仪器制造有限公司 表 3 制备MIP和NIP的组分材料及含量
Table 3. Components and contents for preparing MIP and NIP
聚合物 模板分子类型 模板分子/mmol 功能单体/mmol 交联剂(EDGMA)/mmol 引发剂(AIBN)/mg 致孔剂(乙腈)/mL 温度/℃ 吸附量/(mg·g-1) 印迹因子 分配常数/(g·mL-1) MIP1 胆固醇 0.5 2 10 160.5 60 60 0.670 2.778 0.183 MIP2 β-谷固醇 0.5 2 10 160.5 60 60 0.779 3.231 0.226 MIP3 去氧胆酸 0.5 2 10 160.5 60 60 0.877 3.638 0.270 MIP4 去氧胆酸 0.5 2 10 160.5 60 50 0.418 MIP5 去氧胆酸 0.5 2 10 160.5 60 70 0.695 NIP 2 10 160.5 60 60 0.241 0.053 表 4 模板分子与功能单体的最大理论吸光度与实际吸光度差值
Table 4. Difference between the maximum theoretical absorbance and the actual absorbance of template molecules and functional monomers
模板分子 功能单体 AA MAA MMA AM 胆固醇 1.063 08 0.847 14 0.861 46 0.718 72 β-谷固醇 0.908 60 0.668 06 0.621 24 0.368 06 去氧胆酸 0.298 62 0.133 27 0.095 51 0.060 29 -
[1] SHIRNESHAN G, BAKHTIARI A R, MEMARIANI M. Distribution and origins of n-alkanes, hopanes, and steranes in rivers and marine sediments from southwest Caspian coast, Iran: implications for identifying petroleum hydrocarbon inputs[J]. Environmental Science and Pollution Research, 2016, 23(17): 17484-17495. doi: 10.1007/s11356-016-6825-8 [2] LIU Shiju, GAO Gang, JIN Jun, et al. Source rock with high abundance of C28 regular sterane in typical brackish-saline lacustrine sediments: biogenic source, depositional environment and hydrocarbon generation potential in Junggar Basin, China[J]. Journal of Petroleum Science and Engineering, 2022, 208: 109670. doi: 10.1016/j.petrol.2021.109670 [3] 万涛, 张洪安, 张宝君, 等. C29重排谷甾烷在油源对比研究中的应用: 以银额盆地查干凹陷为例[J]. 断块油气田, 2021, 28(2): 173-178. https://www.cnki.com.cn/Article/CJFDTOTAL-DKYT202102007.htmWAN Tao, ZHANG Hongan, ZHANG Baojun, et al. The application of C29 rearranged sitostane in oil source correlation research: taking Chagan Depression of Yingen-Ejinaqi Basin for example[J]. Fault-Block Oil and Gas Field, 2021, 28(2): 173-178. https://www.cnki.com.cn/Article/CJFDTOTAL-DKYT202102007.htm [4] KASHIRTSEV V A, DOLZHENKO K V, FOMIN A N, et al. Hydrocarbon composition of bitumen from deeply buried terrestrial organic matter (zone of apocatagenesis)[J]. Russian Geology and Geophysics, 2017, 58(6): 702-710. doi: 10.1016/j.rgg.2016.03.018 [5] MEN Jiying, DONG Chengya, SHI Hongxing, et al. Surface molecular imprinted membranes as a "gate" for selective transdermal release of chiral drug amlodipine[J]. Journal of Membrane Science, 2022, 664: 121059. doi: 10.1016/j.memsci.2022.121059 [6] LI Aimin, HUANG Xiaolan, YAN Ling, et al. Pseudo-template molecularly imprinted polymeric fiber solid-phase microextraction coupled to gas chromatography for ultrasensitive determination of 2, 4, 6-trihalophenol disinfection by-products[J]. Journal of Chromatography A, 2022, 1678: 463322. doi: 10.1016/j.chroma.2022.463322 [7] RAMANAVICIUS S, SAMUKAITE-BUBNIENE U, RATAUTAITE V, et al. Electrochemical molecularly imprinted polymer based sensors for pharmaceutical and biomedical applications (review)[J]. Journal of Pharmaceutical and Biomedical Analysis, 2022, 215: 114739. doi: 10.1016/j.jpba.2022.114739 [8] WANG Xingguo, LIU Zhixiang, LU Jian, et al. Highly selective membrane for efficient separation of environmental determinands: enhanced molecular imprinting in polydopamine-embedded porous sleeve[J]. Chemical Engineering Journal, 2022, 449: 137825. doi: 10.1016/j.cej.2022.137825 [9] WANG Xuemei, HUANG Pengfei, MA Xiaomin, et al. Enhanced in-out-tube solid-phase microextraction by molecularly imprinted polymers-coated capillary followed by HPLC for Endocrine Disrupting Chemicals analysis[J]. Talanta, 2019, 194: 7-13. doi: 10.1016/j.talanta.2018.10.027 [10] YUAN Ya, WANG Yuzhi, HUANG Meidong, et al. Development and characterization of molecularly imprinted polymers for the selective enrichment of podophyllotoxin from traditional Chinese medicines[J]. Analytica Chimica Acta, 2011, 695(1/2): 63-72. http://www.sciencedirect.com/science/article/pii/S0003267011005290 [11] KARIM K, BRETON F, ROUILLON R, et al. How to find effective functional monomers for effective molecularly imprinted polymers?[J]. Advanced Drug Delivery Reviews, 2005, 57(12): 1795-1808. doi: 10.1016/j.addr.2005.07.013 [12] 银珍红, 陈小明. 2, 4-二氯苯氧乙酸分子印迹整体柱的制备、表征及色谱性能研究[J]. 分析测试学报, 2009, 28(8): 949-953. https://www.cnki.com.cn/Article/CJFDTOTAL-TEST200908018.htmYING Zhenhong, CHEN Xiaoming. Synthesis and characterization of 2, 4-dichlorophenoxyacetic acid molecularly imprinted monolithic column and its chromatographic property[J]. Journal of Instrumental Analysis, 2009, 28(8): 949-953. https://www.cnki.com.cn/Article/CJFDTOTAL-TEST200908018.htm [13] MA Xingbin, LIN Hongling, ZHANG Jiyu, et al. Preparation and characterization of dummy molecularly imprinted polymers for separation and determination of farrerol from Rhododendronaganniphum using HPLC[J]. Green Chemistry Letters and Reviews, 2018, 11(4): 513-522. doi: 10.1080/17518253.2018.1541481 [14] 李璐, 周刘梅, 解新安, 等. 溴氰菊酯农药残留检测的分子印迹预聚体系筛选及吸附性能[J]. 农业工程学报, 2019, 35(1): 269-277. https://www.cnki.com.cn/Article/CJFDTOTAL-NYGU201901034.htmLI Lu, ZHOU Liumei, XIE Xinan, et al. Screening of molecularly imprinted pre-assembly system for detection of deltamethrin pesticide residues and its specific adsorption properties[J]. Transactions of the Chinese Society of Agricultural Engineering, 2019, 35(1): 269-277. https://www.cnki.com.cn/Article/CJFDTOTAL-NYGU201901034.htm [15] WANG Liping, SHE Xuhui, CHEN Zhi, et al. Preparation and characterization of a chiral molecularly imprinted polymer with a novel functional monomer for controlled release of S-sulpiride[J]. International Journal of Pharmaceutics, 2021, 601: 120526. doi: 10.1016/j.ijpharm.2021.120526 [16] 高博, 杨宏伟, 宋文琦, 等. 分子模拟辅助设计L-苯丙氨酸分子印迹聚合物及其性能研究[J]. 化学通报, 2019, 82(3): 251-257. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201903010.htmGAO Bo, YANG Hongwei, SONG Wenqi, et al. Molecular simulation-aided design of L-phenylalanine-imprinted polymers and its properties[J]. Chemistry, 2019, 82(3): 251-257. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201903010.htm [17] SÁNCHEZ-GONZÁLEZ J, PEÑA-GALLEGO Á, SANMARTÍN J, et al. NMR spectroscopy for assessing cocaine-functional monomer interactions when preparing molecularly imprinted polymers[J]. Microchemical Journal, 2019, 147: 813-817. doi: 10.1016/j.microc.2019.03.088 [18] 杨俊, 朱晓兰, 苏庆德, 等. 可天宁印迹聚合物分子识别特性的光谱与XPS研究[J]. 光谱学与光谱分析, 2007, 27(6): 1152-1155. https://www.cnki.com.cn/Article/CJFDTOTAL-GUAN200706031.htmYANG Jun, ZHU Xiaolan, SU Qingde, et al. Spectroscopy and XPS studies on molecular recognition of a molecularly imprinted cotinine-specific polymer[J]. Spectroscopy and Spectral Analysis, 2007, 27(6): 1152-1155. https://www.cnki.com.cn/Article/CJFDTOTAL-GUAN200706031.htm [19] WADIE M, ABDEL-MOETY EM, REZK M R, et al. Electro-polymerized poly-methyldopa as a novel synthetic mussel-inspired molecularly imprinted polymeric sensor for darifenacin: computational and experimental study[J]. Applied Materials Today, 2022, 29: 101595. doi: 10.1016/j.apmt.2022.101595 [20] GARCIA L L C, FIGUEIREDO-FILHO L C S, OLIVEIRA G G, et al. Square-wave voltammetric determination of paraquat using a glassy carbon electrode modified with multiwalled carbon nanotubes within a dihexadecyl hydrogen phosphate (DHP) film[J]. Sensors and Actuators B: Chemical, 2013, 181: 306-311. doi: 10.1016/j.snb.2013.01.091 [21] 林秋明, 何建峰, 刘岚, 等. 不同功能单体合成的分子印迹聚合物识别性能的研究[J]. 化学研究与应用, 2007, 19(10): 1084-1088. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ200710005.htmLIN Qiuming, HE Jianfeng, LIU Lan, et al. Study on the effect of recognized characteristics of quercetin imprinted polymers with different functional monomers[J]. Chemical Research and Application, 2007, 19(10): 1084-1088. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ200710005.htm [22] 朱淮武. 有机分子结构波谱解析[M]. 北京: 化学工业出版社, 2005.ZHU Huaiwu. Organic molecular structure spectra analysis[M]. Beijing: Chemical Industry Press, 2005. [23] 张晓. 番红花红T分子印迹聚合物的制备及其性能研究[D]. 兰州: 兰州交通大学, 2021.ZHANG Xiao. Preparation and properties of safranin T molecular imprinted polymer[D]. Lanzhou: Lanzhou Jiaotong University, 2021. [24] 刘婷婷, 卢春霞. 齐墩果酸分子印迹体系优化[J]. 江苏农业科学, 2021, 49(2): 139-145. https://www.cnki.com.cn/Article/CJFDTOTAL-JSNY202102025.htmLIU Tingting, LU Chunxia. Optimization of molecularly imprinted system for oleanolic acid[J]. Jiangsu Agricultural Sciences, 2021, 49(2): 139-145. https://www.cnki.com.cn/Article/CJFDTOTAL-JSNY202102025.htm [25] 张孝刚, 朱秋劲, 胡萍. 三聚氰胺分子印迹预组装体系紫外光谱研究[J]. 食品科学, 2011, 32(21): 128-132. https://www.cnki.com.cn/Article/CJFDTOTAL-SPKX201121025.htmZHANG Xiaogang, ZHU Qiujin, HU Ping. Ultraviolet spectroscopic investigations into melamine molecular imprinting pre-assembly system[J]. Food Science, 2011, 32(21): 128-132. https://www.cnki.com.cn/Article/CJFDTOTAL-SPKX201121025.htm [26] CHEN Changbao, CHEN Yanjun, ZHOU Jie, et al. A 9-vinyladenine-based molecularly imprinted polymeric membrane for the efficient recognition of plant hormone 1H-indole-3-acetic acid[J]. Analytica Chimica Acta, 2006, 569(1/2): 58-65. http://www.sciencedirect.com/science/article/pii/S0003267006006362 -





 下载:
下载:














 苏公网安备32021102000780号
苏公网安备32021102000780号