Chromatography-vacuum low temperature method of methane enrichment and isotopic fractionation in gas samples
-
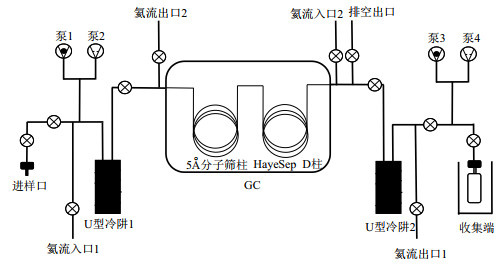
摘要: 甲烷(CH4)团簇同位素分析在气候变化、能源勘探和行星生命等领域中发挥了重要作用。样品中CH4的纯度直接了影响高分辨质谱团簇同位素分析的精度和准确性。针对气样中CH4组分的富集纯化难题,根据气相色谱(GC)组分分离原理,实时监测组分峰形,进一步优化了载气线速、进样量等条件。同时,通过外标法量化回收率,GC组分分析验证纯度,保证纯化的有效性。通过优化色谱—真空低温富集制备方法,确定了IBEX系统载气最佳线速为12 mL/min,CH4进样量需小于12 mL等实验条件,可视化GC峰形确保CH4峰与相邻N2干扰峰基本分离,实现了CH4单组分的高纯富集。当气样中CH4含量小于70%而空气含量较高时,需要进行二次纯化以提高CH4纯度。讨论了5Å分子筛等吸附剂在纯化过程中可能引起CH4同位素分馏的原因,并通过适当延长CH4收集时间来消除5Å分子筛干扰。目前,该方法单次纯化过程约90 min,CH4的回收率和纯度分别为90.1%~95.7%和97.3%~98.9%,对同位素组成(δ13CVPDB和δDVSMOW、Δ13CH3D和Δ12CH2D2)的差异均小于质谱仪的分析误差,几乎可以忽略不计。Abstract: Methane (CH4) clumped isotope analysis plays a crucial role in the fields of climate change, energy exploration, and planetary research. The purity of CH4 in samples directly affects the precision and accuracy of high-resolution mass spectrometry in clumped isotope analysis. Addressing the challenge associated with enriching and purifying CH4 components in gas samples, this study optimized conditions such as carrier gas line speed and sample injection volume based on the principles of gas chromatography (GC) component separation, with real-time monitoring of component peak shapes. Additionally, the recovery rate was quantified using an external standard method and purity was verified through GC component analysis to ensure the effectiveness of the purification process. By optimizing the chromatography-vacuum low-temperature enrichment preparation method, the optimal carrier gas line speed for the IBEX system was determined to be 12 mL/min, with a CH4 injection volume less than 12 mL. This facilitated visualization of GC peak shapes, thus ensured that the CH4 peak was essentially separated from the adjacent N2 interference peak, achieving high-purity enrichment of the CH4 single component. When the CH4 content in gas samples was less than 70% and the air content was high, secondary purification was required to improve CH4 purity. The causes of CH4 isotopic fractionation during purification using adsorbents like 5Å molecular sieves were discussed, and extending the CH4 collection time was proposed to eliminate the interference from the 5Å molecular sieve. Currently, this method requires approximately 90 min for a single purification process, with CH4 recovery and purity ranging from 90.1% to 95.7% and 97.3% to 98.9%, respectively. The differences in isotopic composition (δ13CVPDB and δDVSMOW, Δ13CH3D, and Δ12CH2D2) are all less than the analytical error of the mass spectrometer, making them almost negligible.
-
Key words:
- CH4 /
- gas chromatography /
- purity /
- recovery rate /
- isotopic fractionation
-
表 1 甲烷气样SG-1纯化前后同位素组成对比
Table 1. Comparison of isotopic composition of methane gas sample SG-1 before and after purification
δ13CVPDB/‰ δDVSMOW/‰ Δ13CH3D/‰ Δ12CH2D2/‰ 样品数 纯化前 -43.23 -182.78 2.65 2.14 5 纯化后 -43.30 -182.82 2.91 1.50 5 表 2 通过改变载气线速纯化气样后甲烷回收率及纯度数据
Table 2. Recovery and purity data of methane after purification of gas sample by varying carrier gas line speed
样品 体积/mL 柱温/℃ 载气流速/(mL/min) 峰面积 回收率/% 纯度/% O2 N2 CH4 SG-1 6 30 30 80.8 310.2 22 372.9 82.1 98.3 MG-20% 6 30 30 183.4 3 020.3 18 167.3 83.6 85.0 SG-1 6 30 20 99.1 347.4 23 279.6 85.5 98.1 MG-20% 6 30 20 187.2 1 804.5 18 913.2 87.1 90.5 SG-1 6 30 15 145.9 408.3 24 157.7 88.7 97.8 MG-20% 6 30 15 195.7 1 076.7 19 235.5 88.6 93.8 SG-1 6 30 12 112.2 353.6 25 940.1 95.2 98.2 MG-20% 6 30 12 177.3 415.1 20 831.4 95.9 97.2 SG-1 6 30 10 136.8 348.1 25 673.3 94.2 98.1 MG-20% 6 30 10 189.5 394.4 20 374.8 93.8 97.2 SG-1 9 30 12 92.7 384.2 38 221.7 93.1 98.8 SG-2 9 30 12 109.8 401.1 18 284.2 95.0 97.3 SG-1 12 30 12 112.4 434.1 47 589.2 86.8 98.9 SG-2 12 30 12 87.3 397.5 24 442.7 94.8 98.1 SG-1 18 30 12 124.4 468.3 70 179.5 85.1 99.2 SG-2 18 30 12 97.6 445.8 36 950.7 95.1 98.6 MG-10% 8 30 12 128.4 331.3 30 912.2 94.4 98.5 MG-20% 8 30 12 133.6 358.2 27 098.3 93.2 98.2 MG-30% 8 30 12 115.1 3 197.8 23 289.8 91.7 87.5 MG-30%-2nd 8 30 12 88.3 405.6 22 868.9 90.0 97.9 MG-50% 8 30 12 150.9 5 266.9 16 851.1 93.4 75.7 MG-50%-2nd 8 30 12 73.6 409.4 16 491.9 91.4 97.2 注:样品名中,百分比表示气样中N2含量,“2nd”表示二次纯化。 表 3 甲烷不充分回收时同位素组成
Table 3. Isotopic composition of CH4 in inadequate recovery
气样 体积/mL 采样时间/min 回收率/% 纯度/% δ13CVPDB/‰ SG-1 6 5 36.3 98.1 -41.52 SG-1 6 10 54.0 98.6 -42.22 SG-1 6 17 69.4 98.4 -42.68 SG-1 6 30 88.6 97.8 -42.89 SG-1 6 40 90.2 98.0 -43.02 SG-1 6 50 94.1 97.5 -43.39 注:SG-1初始同位素组成δ13CVPDB-initial=-43.26‰。 -
[1] DOUGLAS P M J, STOLPER D A, SMITH D A, et al. Diverse origins of arctic and subarctic methane point source emissions identified with multiply-substituted isotopologues[J]. Geochimica et Cosmochimica Acta, 2016, 188: 163-188. doi: 10.1016/j.gca.2016.05.031 [2] BEAUDRY P, STEFÁNSSON A, FIEBIG J, et al. High temperature generation and equilibration of methane in terrestrial geothermal systems: evidence from clumped isotopologues[J]. Geochimica et Cosmochimica Acta, 2021, 309: 209-234. doi: 10.1016/j.gca.2021.06.034 [3] GIUNTA T, YOUNG E D, LABIDI J, et al. Extreme methane clumped isotopologue bio-signatures of aerobic and anaerobic methanotrophy: insights from the Lake Pavin and the Black Sea sediments[J]. Geochimica et Cosmochimica Acta, 2022, 338: 34-53. doi: 10.1016/j.gca.2022.09.034 [4] YOUNG E D, KOHL I E, SHERWOOD LOLLAR B, et al. The relative abundances of resolved l2CH2D2 and 13CH3D and mechanisms controlling isotopic bond ordering in abiotic and biotic methane gases[J]. Geochimica et Cosmochimica Acta, 2017, 203: 235-264. doi: 10.1016/j.gca.2016.12.041 [5] 马东民, 王馨, 滕金祥, 等. 镜煤和暗煤与甲烷界面作用实验研究: 以民和盆地低阶煤为例[J]. 油气藏评价与开发, 2022, 12(4): 556-563. https://www.cnki.com.cn/Article/CJFDTOTAL-KTDQ202204002.htmMA Dongmin, WANG Xin, TENG Jinxiang, et al. Experimental study on interfacial interaction between methane and vitrinite and durain: a case study of bituminous coal in Minhe Basin[J]. Petroleum Reservoir Evaluation and Development, 2022, 12(4): 556-563. https://www.cnki.com.cn/Article/CJFDTOTAL-KTDQ202204002.htm [6] 杨琴, 黄亮, 周文, 等. 深层页岩伊利石孔隙中甲烷吸附相密度特征[J]. 断块油气田, 2023, 30(5): 799-807. https://www.cnki.com.cn/Article/CJFDTOTAL-DKYT202305012.htmYANG Qin, HUANG Liang, ZHOU Wen, et al. Adsorption phase density characteristics of methane in illite pores of deep shale[J]. Fault-Block Oil and Gas Field, 2023, 30(5): 799-807. https://www.cnki.com.cn/Article/CJFDTOTAL-DKYT202305012.htm [7] 毛港涛, 李治平, 王凯, 等. 全可视化双反应釜内甲烷水合物生成与分解特征研究[J]. 特种油气藏, 2023, 30(3): 73-80. https://www.cnki.com.cn/Article/CJFDTOTAL-TZCZ202303009.htmMao Gangtao, Li Zhiping, Wang Kai, et al. Study on the generation and decomposition characteristics of methane hydrate in Fully Visible Dual Reactor[J]. Special Oil & Gas Reservoirs, 2023, 30(3): 73-80. https://www.cnki.com.cn/Article/CJFDTOTAL-TZCZ202303009.htm [8] 史利燕, 李卫波, 康琴琴, 等. CH4-煤吸附/解吸过程视电阻率变化的实验研究[J]. 油气藏评价与开发, 2022, 12(4): 572-579. https://www.cnki.com.cn/Article/CJFDTOTAL-KTDQ202204004.htmSHI Liyan, LI Weibo, KANG Qinqin, et al. Experimental study on variation of apparent resistivity in CH4-coal adsorption/desorption process[J]. Petroleum Reservoir Evaluation and Development, 2022, 12(4): 572-579. https://www.cnki.com.cn/Article/CJFDTOTAL-KTDQ202204004.htm [9] 张添锦, 王延峰, 李军, 等. 注CO2提高页岩吸附甲烷采收率核磁共振实验[J]. 特种油气藏, 2023, 30(5): 113-120. doi: 10.3969/j.issn.1006-6535.2023.05.015Zhang Tianjin, Wang Yanfeng, Li Jun, et al. Nuclear magnetic resonance experiment for enhanced recovery af adsorbed methane from shale through carbon dioxide injection[J]. Special Oil & Gas Reservoirs, 2023, 30(5): 113-120. doi: 10.3969/j.issn.1006-6535.2023.05.015 [10] WHITICAR M J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane[J]. Chemical Geology, 1999, 161(1/3): 291-314. [11] EILER J M. A practical guide to clumped isotope geochemistry[J]. Geochimica et Cosmochimica Acta, 2006, 70(S18): A157. [12] EILER J M. "Clumped-isotope" geochemistry—The study of naturally-occurring, multiply-substituted isotopologues[J]. Earth and Planetary Science Letters, 2007, 262(3/4): 309-327. [13] EILER J M, CLOG M, MAGYAR P, et al. A high-resolution gas-source isotope ratio mass spectrometer[J]. International Journal of Mass Spectrometry, 2013, 335: 45-56. doi: 10.1016/j.ijms.2012.10.014 [14] YOUNG E D, RUMBLE Ⅲ D, FREEDMAN P, et al. A large-radius high-mass-resolution multiple-collector isotope ratio mass spectrometer for analysis of rare isotopologues of O2, N2, CH4 and other gases[J]. International Journal of Mass Spectrometry, 2016, 401: 1-10. doi: 10.1016/j.ijms.2016.01.006 [15] ONO S, WANG D T, GRUEN D S, et al. Measurement of a doubly substituted methane isotopologue, 13CH3D, by tunable infrared laser direct absorption spectroscopy[J]. Analytical Chemistry, 2014, 86(13): 6487-6494. doi: 10.1021/ac5010579 [16] GONZALEZ Y, NELSON D D, SHORTER J H, et al. Precise measurements of 12CH2D2 by tunable infrared laser direct absorption spectroscopy[J]. Analytical Chemistry, 2019, 91(23): 14967-14974. doi: 10.1021/acs.analchem.9b03412 [17] GILBERT A, YAMADA K, YOSHIDA N. Exploration of intramolecular 13C isotope distribution in long chain n-alkanes (C11-C31) using isotopic 13C NMR[J]. Organic Geochemistry, 2013, 62: 56-61. doi: 10.1016/j.orggeochem.2013.07.004 [18] MARTIN G J, GUILLOU C, MARTIN M L, et al. Natural factors of isotope fractionation and the characterization of wines[J]. Journal of Agricultural and Food Chemistry, 1988, 36(2): 316-322. doi: 10.1021/jf00080a019 [19] GILBERT A, SILVESTRE V, SEGEBARTH N, et al. The intramolecular 13C-distribution in ethanol reveals the influence of the CO2-fixation pathway and environmental conditions on the site-specific 13C variation in glucose[J]. Plant, Cell & Environment, 2011, 34(7): 1104-1112. [20] GILBERT A. The organic isotopologue frontier[J]. Annual Review of Earth and Planetary Sciences, 2021, 49(1): 435-464. doi: 10.1146/annurev-earth-071420-053134 [21] STOLPER D A, SESSIONS A L, FERREIRA A A, et al. Combined 13C-D and D-D clumping in methane: methods and preliminary results[J]. Geochimica et Cosmochimica Acta, 2014, 126: 169-191. doi: 10.1016/j.gca.2013.10.045 [22] STOLPER D A, LAWSON M, DAVIS C L, et al. Formation temperatures of thermogenic and biogenic methane[J]. Science, 2014, 344(6191): 1500-1503. doi: 10.1126/science.1254509 [23] WANG D T, GRUEN D S, LOLLAR B S, et al. Nonequilibrium clumped isotope signals in microbial methane[J]. Science, 2015, 348(6233): 428-431. doi: 10.1126/science.aaa4326 [24] WANG D T, WELANDER P V, ONO S. Fractionation of the methane isotopologues 13CH4, 12CH3D, and 13CH3D during aerobic oxidation of methane by Methylococcus capsulatus (Bath)[J]. Geochimica et Cosmochimica Acta, 2016, 192: 186-202. doi: 10.1016/j.gca.2016.07.031 [25] JENNINGS W. Analytical gas chromatography[M]. Orlando: Academic Press Inc., 1987. [26] CHEN Zhigang, YIN Xijie, ZHOU Youping. Effects of GC temperature and carrier gas flow rate on on-line oxygen isotope measurement as studied by on-column CO injection[J]. Journal of Mass Spectrometry, 2015, 50(8): 1023-1030. doi: 10.1002/jms.3617 [27] WERRES T, SCHMIDT T C, TEUTENBERG T. The influence of injection volume on efficiency of microbore liquid chromatography columns for gradient and isocratic elution[J]. Journal of Chromatography A, 2021, 1641: 461965. doi: 10.1016/j.chroma.2021.461965 [28] STRАPOĆD, SCHIMMELMANN A, MASTALERZ M. Carbon isotopic fractionation of CH4 and CO2 during canister desorption of coal[J]. Organic Geochemistry, 2006, 37(2): 152-164. doi: 10.1016/j.orggeochem.2005.10.002 [29] XIA Xinyu, TANG Yongchun. Isotope fractionation of methane during natural gas flow with coupled diffusion and adsorption/desorption[J]. Geochimica et Cosmochimica Acta, 2012, 77: 489-503. doi: 10.1016/j.gca.2011.10.014 [30] 苏现波, 陈润, 林晓英, 等. 煤吸附13CH4与12CH4的特性曲线及其应用[J]. 煤炭学报, 2007, 32(5): 539-543. doi: 10.3321/j.issn:0253-9993.2007.05.021SU Xianbo, CHEN Run, LIN Xiaoying, et al. The adsorption characteristic curves of 13CH4 and 12CH4 on coal and its application[J]. Journal of China Coal Society, 2007, 32(5): 539-543. doi: 10.3321/j.issn:0253-9993.2007.05.021 [31] WANG Xiaofeng, LI Xiaofu, WANG Xiangzeng, et al. Carbon isotopic fractionation by desorption of shale gases[J]. Marine and Petroleum Geology, 2015, 60: 79-86. doi: 10.1016/j.marpetgeo.2014.11.003 [32] MASON E A, KRONSTADT B. Graham's laws of diffusion and effusion[J]. Journal of Chemical Education, 1967, 44(12): 740. doi: 10.1021/ed044p740 [33] GUNTER B D, GLEASON J D. Isotope fractionation during gas chromatographic separations[J]. Journal of Chromatographic Science, 1971, 9(3): 191-192. doi: 10.1093/chromsci/9.3.191 [34] CUI Xiaojun, MARC BUSTIN R, DIPPLE G. Selective transport of CO2, CH4, and N2 in coals: insights from modeling of experimental gas adsorption data[J]. Fuel, 2004, 83(3): 293-303. doi: 10.1016/j.fuel.2003.09.001 [35] WANG Xiaofeng, LIU Peng, MENG Qiang, et al. Physical selectivity on isotopologues of gaseous alkanes by shale pore network: evidence from dynamic adsorption process of natural gas[J]. Journal of Natural Gas Science and Engineering, 2022, 97: 104252. doi: 10.1016/j.jngse.2021.104252 [36] CRANK J. The mathematics of diffusion[M]. 2nd ed. Oxford: Clarendon Press, 1975. [37] 程付启, 金强. 成藏后天然气组分与同位素的分馏效应研究[J]. 天然气地球科学, 2005, 16(4): 522-525. doi: 10.3969/j.issn.1672-1926.2005.04.022CHENG Fuqi, JIN Qiang. Composition and isotope fractionations of accumulated natural gas and their significance[J]. Natural Gas Geoscience, 2005, 16(4): 522-525. doi: 10.3969/j.issn.1672-1926.2005.04.022 -





 下载:
下载:





 苏公网安备32021102000780号
苏公网安备32021102000780号