A simultaneous determination method for diamondoids and thiadiamondoids in condensate oil and its geological significance: taking condensate oil from central Tarim Basin as an example
-
摘要: 原油中的金刚烷类和硫代金刚烷类化合物具有相似的类金刚石笼状结构,可以反映地质过程中的热裂解作用和热化学硫酸盐还原反应(TSR)作用,因此两者的同步检出,不仅可以提高样品的分析测试效率和硫代金刚烷类化合物的定量结果准确性,还可以为样品提供更加可靠和广泛的地球化学分析解释。该文利用气相色谱-三重四级杆串联质谱仪(GC-MS-MS),确定了目标化合物的母离子和子离子、扫描时间、碰撞能等仪器参数,建立了塔里木盆地塔中地区凝析油中金刚烷类化合物和硫代金刚烷类化合物的同步定量检测方法,并通过定量检测结果发现,塔中地区金刚烷类化合物的含量虽有差异,但是成熟度相近,都处于过成熟阶段,且部分样品曾经历过TSR作用。Abstract: Diamondoids and thiadiamondoids in crude oil samples have the similar diamond-like cage structures, which can reflect the thermal cracking and thermochemical sulfate reduction(TSR) effect during geological processes. Therefore, the simultaneous monitoring of diamondoids and thiadiamondoids cannot only improve the efficiency of sample analysis and the accuracy of quantitative results of thiadiamondoids, but also provide more reliable and extensive geochemical interpretation for the samples. In this paper, a simultaneous quantitative detection method of diamondoids and thiadiamondoids was proposed by the means of gas chromatography-triple quadrupole mass spectrometer (GC-MS-MS). By determining the parameters of precursor and product ions, scanning time and collision energy of the target compound, a simultaneous quantitative detection method was established for diamondoids and thiadiamondoids in condensate from the central Tarim Basin. Results showed that despite the contents of diamondoids of oils from the central Tarim Basin were different, the maturity degree on the other hand, were comparable as over mature, and some samples have experienced TSR.
-
Key words:
- diamondoids /
- thiadiamondoids /
- maturity /
- thermochemical sulfate reduction (TSR) /
- central Tarim /
- Tarim Basin
-
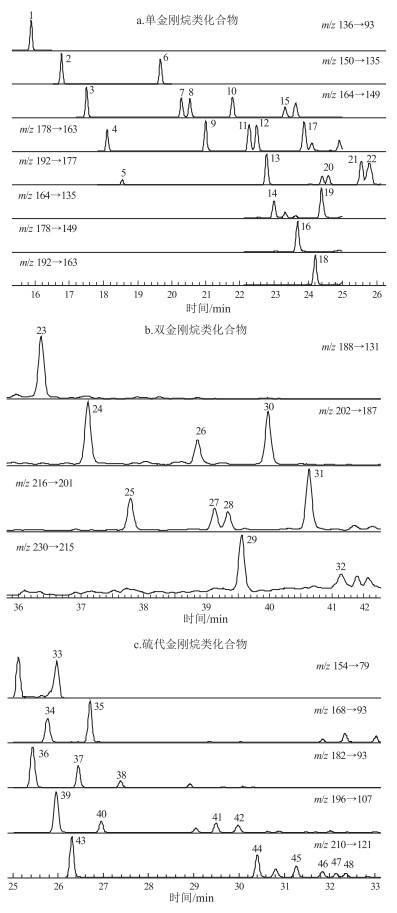
图 1 塔里木盆地塔中地区样品TZ103中单金刚烷类化合物(a)、双金刚烷类化合物(b)、2-硫代单金刚烷类化合物(c)的色谱图
图中峰号代表的化合物见表 3。
Figure 1. Chromatograms of adamantanes (a), diamantanes (b) and thiaadamantanes (c) in sample TZ103 from central Tarim Basin
表 1 塔里木盆地塔中地区凝析油样品基本信息
Table 1. Basic information of condensate oil samples from central Tarim Basin
井号 层位 井深/m 密度/(kg·m-3) 黏度/(mm2·s-1) 含硫量/(mg·L-1) TZ103 C 3 755.0~3 756.5 835.76 2.483 2 380 TZ83-3 O1y 5 580.00~5 648.53 807.57 3.041 2 420 TZ86 O 6 273~6 320 820.75 4.530 3 170 TZ451 C 6 090.50~6 297.62 802.86 2.743 1 350 TZ823 5 369~5 490 824.77 5.032 1 750 TZ82-1H O1y 5 247.36~6 280.00 809.01 3.270 1 470 TZ162 O 5 048~5 070 835.38 3.851 1 440 玛4-H4 O 2 086~2 840 785.81 1.103 2 240 TZ62-H14 O3z 4 764~5 163 789.82 1.959 1 160 表 2 金刚烷类化合物及硫代金刚烷类化合物同时检测的参数设置
Table 2. Parameter setting for simultaneous detection of diamondoids and thiadiamondoids
片段 时长/min 扫描时间/s 母离子(m/z) 子离子(m/z) 碰撞能/V 1 16.5 0.500 136 93.2 13 2 0.70 0.500 150 135 6 3 0.60 0.500 164 149 6 4 1.60 0.250 178 163 6 192 177 6 5 0.60 0.500 150 135 9 6 1.60 0.150 164 149 8 178 163 6 196.4 82.1 5 7 0.50 0.500 164 149 6 8 2.90 0.100 164 149 6 164 135 6 178 163 6 178 149 6 192 177 6 192 163 6 9 1.15 0.050 192 177 6 154 79 13 168 93 6 182 93 6 196 107 6 10 9.65 0.050 168 93 6 182 93 6 196 107 6 210 121 6 11 9.20 0.100 188.1 131.3 13 202 187 6 216 201 6 230 215 6 260.7 82.1 7 峰号 化合物 分子式 英文名称 缩写 1 单金刚烷 C10H16 adamantane A 2 1-甲基单金刚烷 C11H18 1-methyladamantane 1-MA 3 1, 3-二甲基单金刚烷 C12H20 1, 3-dimethyladamantane 1, 3-DMA 4 1, 3, 5-三甲基单金刚烷 C13H22 1, 3, 5-trimethyladamantane 1, 3, 5-TMA 5 1, 3, 5, 7-四甲基单金刚烷 C14H24 1, 3, 5, 7-tetramethyladamantane 1, 3, 5, 7-TeMA 6 2-甲基单金刚烷 C11H18 2-methyladamantane 2-MA 7 1, 4-二甲基单金刚烷(顺式) C12H20 1, 4-dimethyladamantane(cis) 1, 4-DMA(cis) 8 1, 4-二甲基单金刚烷(反式) C12H20 1, 4-dimethyladamantane(trans) 1, 4-DMA(trans) 9 1, 3, 6-三甲基单金刚烷 C13H22 1, 3, 6-trimethyladamantane 1, 3, 6-TMA 10 1, 2-二甲基单金刚烷 C12H20 1, 2-dimethyladamantane 1, 2-DMA 11 1, 3, 4-三甲基单金刚烷(顺式) C13H22 1, 3, 4-trimethyladamantane(cis) 1, 3, 4-TMA(cis) 12 1, 3, 4-三甲基单金刚烷(反式) C13H22 1, 3, 4-trimethyladamantane(trans) 1, 3, 4-TMA(trans) 13 1, 2, 5, 7-四甲基单金刚烷 C14H24 1, 2, 5, 7-tetramethyladamantane 1, 2, 5, 7-TeMA 14 1-乙基单金刚烷 C12H20 1-ethyladamantane 1-EA 15 2, 6-+2, 4-二甲基单金刚烷 C12H20 2, 6+2, 4-dimethyladamantane 2, 6+2, 4-DMA 16 1-乙基-3-甲基单金刚烷 C13H22 1-E-3-methyladamantane 1-E-3-MA 17 1, 2, 3 -三甲基单金刚烷 C13H22 1, 2, 3-trimethyladamantane 1, 2, 3-TMA 18 1-乙基-3, 5-二甲基单金刚烷 C14H24 1-E-3, 5-dimethyladamantane 1-E-3, 5-DMA 19 2-乙基单金刚烷 C12H20 2-ethyladamantane 2-EA 20 1, 3, 5, 6-四甲基单金刚烷 C14H24 1, 3, 5, 6-tetramethyladamantane 1, 3, 5, 6-TeMA 21 1, 2, 3, 5-四甲基单金刚烷 C14H24 1, 2, 3, 5, -tetramethyladamantane 1, 2, 3, 5-TeMA 22 1-乙基-3, 5, 7-三甲基双金刚烷 C15H26 1-E-3, 5, 7-trimethyladamantane 1-E-3, 5, 7-TMA 23 双金刚烷 C14H20 diamantane D 24 4-甲基双金刚烷 C15H22 4-methyldiamantane 4-MD 25 4, 9-二甲基双金刚烷 C16H24 4, 9-dimethyldiamantane 4, 9-DMD 26 1-甲基双金刚烷 C15H22 1-methyldiamantane 1-MD 27 1, 4-+2, 4-二甲基双金刚烷 C16H24 1, 4+2, 4-dimethyldiamantane 1, 4+2, 4-DMD 28 4, 8-二甲基双金刚烷 C16H24 4, 8-dimethyldiamantane 4, 8-DMD 29 1, 4, 9-三甲基双金刚烷 C17H26 1, 4, 9-trimethyldiamantane 1, 4, 9-TMD 30 3-甲基双金刚烷 C15H22 3-methyldiamantane 3-MD 31 3, 4-二甲基双金刚烷 C16H24 3, 4-dimethyldiamantane 3, 4-DMD 32 3, 4, 9-三甲基双金刚烷 C16H24 3, 4, 9-trimethyldiamantane 3, 4, 9-TMD 33 2-硫代单金刚烷 C9H14S 2-thiaadamantane TA 34 5-甲基-2-硫代单金刚烷 C10H16S 5-methyl-2-thiaadamantane 5-MTA 35 1-甲基-2-硫代单金刚烷 C10H16S 1-methyl-2-thiaadamantane 1-MTA 36 5, 7-二甲基-2-硫代单金刚烷 C10H16S 5, 7-dimethyl-2-thiaadamantane 5, 7-DMTA 37 1, 5-二甲基-2-硫代单金刚烷 C11H18S 1, 5-dimethyl-2-thiaadamantane 1, 5-DMTA 38 1, 3-二甲基-2-硫代单金刚烷 C11H18S 1, 3-dimethyl-2-thiaadamantane 1, 3-DMTA 39 3, 5, 7-三甲基-2-硫代单金刚烷 C12H20S 3, 5, 7-trimethyl-2-thiaadamantane 3, 5, 7-TMTA 40 1, 5, 7-三甲基-2-硫代单金刚烷 C12H20S 1, 5, 7-trimethyl-2-thiaadamantane 1, 5, 7-TMTA 41 1, 3, 7-三甲基-2-硫代单金刚烷 C12H20S 1, 3, 7-trimethyl-2-thiaadamantane 1, 3, 7-TMTA 42 1, 3, 5-三甲基-2-硫代单金刚烷 C12H20S 1, 3, 5-trimethyl-2-thiaadamantane 1, 3, 5-TMTA 43 1, 3, 5, 7-四甲基-2-硫代单金刚烷 C13H22S 1, 3, 5, 7-tetramethyl-2-thiaadamantane 1, 3, 5, 7-TeMTA 44~48 四甲基-2-硫代单金刚烷 C13H22S tetramethyl-2-thiaadamantanes TeMTAs -
[1] LANDA S, MACHACEK V. Adamantane, a new hydrocarbon extracted from petroleum. Collection czechoslov[J]. Chemical Communications, 1933, 5: 1-5. [2] 付宁, 于晓果, 赵盛蓉. 天然气中金刚烷类化合物的检出及其应用[J]. 石油实验地质, 1998, 20(3): 65-69. doi: 10.11781/sysydz199803267FU Ning, YU Xiaoguo, ZHAO Shengrong. Analysis of diamondoid hydrocarbons in natural gas and its application to Ying Qiong Basin[J]. Experimental Petroleum Geology, 1998, 20(3): 65-69. doi: 10.11781/sysydz199803267 [3] LIN Rui, WILK Z A. Natural occurrence of tetramantane (C22H28), pentamantane (C26H32) and hexamantane (C30H36) in a deep petroleum reservoir[J]. Fuel, 1995, 74(10): 1512-1521. doi: 10.1016/0016-2361(95)00116-M [4] STOUT S A, DOUGLAS G S. Diamondoid hydrocarbons-application in the chemical fingerprinting of natural gas condensate and gasoline[J]. Environmental Forensics, 2004, 5(4): 225-235. doi: 10.1080/15275920490886734 [5] SASSEN R, POST P. Enrichment of diamondoids and 13C in condensate from Hudson Canyon, US Atlantic[J]. Organic Geochemistry, 2008, 39(1): 147-151. doi: 10.1016/j.orggeochem.2007.10.004 [6] BENDER A O, SAID E Z, ABDULSADA A K. Gas chromatographic identification of adamantanes in some Iraqi crude oils[J]. Analyst, 1986, 111(5): 575-576. doi: 10.1039/an9861100575 [7] WINGERT W S. G. C. -M. S. analysis of diamondoid hydrocarbons in Smackover petroleums[J]. Fuel, 1992, 71(1): 37-43. doi: 10.1016/0016-2361(92)90190-Y [8] CHEN Junhong, FU Jiamo, SHENG Guoying, et al. Diamondoid hydrocarbon ratios: novel maturity indices for highly mature crude oils[J]. Organic Geochemistry, 1996, 25(3/4): 179-190. [9] GRICE K, ALEXANDER R, KAGI R I. Diamondoid hydrocarbon ratios as indicators of biodegradation in Australian crude oils[J]. Organic Geochemistry, 2000, 31(1): 67-73. doi: 10.1016/S0146-6380(99)00137-0 [10] DAHL J E, LIU S G, RMK C. Isolation and structure of higher diamondoids, nanometer-sized diamond molecules[J]. Science, 2003, 299(5603): 96-99. doi: 10.1126/science.1078239 [11] AZEVEDO D A, TAMANQUEIRA J B, DIAS J, et al. Multivariate statistical analysis of diamondoid and biomarker data from Brazilian Basin oil samples[J]. Fuel, 2008, 87(10/11): 2122-2130. [12] IMUTA K, OUCHI K. Isolation of adamantane from coal extract[J]. Fuel, 1973, 52(4): 301-302. doi: 10.1016/0016-2361(73)90062-8 [13] ACZEL T, GORBATY M L, MAA P S, et al. Stability of adamantane and its derivatives to coal-liquefaction conditions, and its implications toward the organic structure of coal[J]. Fuel, 1979, 58(3): 228-230. doi: 10.1016/0016-2361(79)90123-6 [14] SCHULZ L K, WILHELMS A, REIN E, et al. Application of diamondoids to distinguish source rock facies[J]. Organic Geochemistry, 2001, 32(3): 365-375. doi: 10.1016/S0146-6380(01)00003-1 [15] WEI Zhibin, MOLDOWAN J, JARVIE D, et al. The fate of diamondoids in coals and sedimentary rocks[J]. Geology, 2006, 34(12): 1013-1016. doi: 10.1130/G22840A.1 [16] DAHL J E, MOLDOWAN J M, PETERS K E, et al. Diamondoid hydrocarbons as indicators of natural oil cracking[J]. Nature, 1999, 399(6731): 54-57. doi: 10.1038/19953 [17] ZHANG Shuichang, HUANG Haiping, XIAO Zhongyao, et al. Geochemistry of Palaeozoic marine petroleum from the Tarim Basin, NW China. Part 2: maturity assessment[J]. Organic Geochemistry, 2005, 36(8): 1215-1225. doi: 10.1016/j.orggeochem.2005.01.014 [18] WEI Zhibin, MOLDOWAN J M, PETERS K E, et al. The abundance and distribution of diamondoids in biodegraded oils from the San Joaquin Valley: implications for biodegradation of diamondoids in petroleum reservoirs[J]. Organic Geochemistry, 2007, 38(11): 1910-1926. doi: 10.1016/j.orggeochem.2007.07.009 [19] WEI Zhibin, MANKIEWICZ P, WALTERS C, et al. Natural occurrence of higher thiadiamondoids and diamondoidthiols in a deep petroleum reservoir in the Mobile Bay gas field[J]. Organic Geochemistry, 2012, 42(2): 121-133. [20] WANG Zhendi, YANG Chun, HOLLEBONE B, et al. Forensic fingerprinting of diamondoids for correlation and differentiation of spilled oil and petroleum products[J]. Environmental Science & Technology, 2006, 40(18): 5636-5646. [21] CAI Chunfang, XIAO Qinlin, FANG Chenchen, et al. The effect of thermochemical sulfate reduction on formation and isomerization of thiadiamondoids and diamondoids in the Lower Paleozoic petroleum pools of the Tarim Basin, NW China[J]. Organic Geochemistry, 2016, 101: 49-62. doi: 10.1016/j.orggeochem.2016.08.006 [22] ZHU Guangyou, ZHANG Ying, WANG Meng, et al. Discovery of high-abundance diamondoids and thiadiamondoids and severe TSR alteration of well ZS1C condensate, Tarim Basin, China[J]. Energy & Fuels, 2018, 32(7): 7383-7392. [23] WEI Zhibin, MOLDOWAN J M, FAGO F, et al. Origins of thiadiamondoids and diamondoidthiols in petroleum[J]. Energy & Fuels, 2007, 21(6): 3431-3436. [24] HANIN S, ADAM P, KOWALEWSKI I, et al. Bridgehead alkylated 2-thiaadamantanes: novel markers for sulfurisation processes occurring under high thermal stress in deep petroleum reservoirs[J]. Chemical Communications, 2002(16): 1750-1751. doi: 10.1039/b203551k [25] BIRCH S, CULLUM T V, DEAN R A, et al. Thiaadamantane[J]. Nature, 1952, 170(4328): 629-630. [26] WANG Meng, ZHU Guangyou, REN Limin, et al. Separation and characterization of sulfur compounds in ultra-deep formation crude oils from Tarim Basin[J]. Energy & Fuels, 2015, 29(8): 4842-4849. [27] ZHU Guangyou, WANG Huitong, WENG Na. TSR-altered oil with high-abundance thiaadamantanes of a deep-buried Cambrian gas condensate reservoir in Tarim Basin[J]. Marine and Petroleum Geology, 2016, 69: 1-12. doi: 10.1016/j.marpetgeo.2015.10.007 [28] 晏继发, 马安来, 李杰豪, 等. 原油金刚烷类化合物2种常用检测方法的对比[J]. 天然气地球科学, 2020, 31(3): 436-445. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX202003014.htmYAN Jifa, MA Anlai, LI Jiehao, et al. Comparison of two determination methods for diamondoids in crude oil[J]. Natural Gas Geoscience, 2020, 31(3): 436-445. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX202003014.htm [29] 王汇彤, 翁娜, 张水昌, 等. 石油样品中金刚烷类化合物的定量分析新方法[J]. 石油实验地质, 2019, 41(3): 443-450. doi: 10.11781/sysydz201903443WANG Huitong, WENG Na, ZHANG Shuichang, et al. A novel method for quantitative analysis of diamondoids in petroleum samples[J]. Petroleum Geology & Experiment, 2019, 41(3): 443-450. doi: 10.11781/sysydz201903443 [30] LIANG Qianyong, XIONG Yongqiang, FANG Chenchen, et al. Quantitative analysis of diamondoids in crude oils using gas chromatography-triple quadrupole mass spectrometry[J]. Organic Geochemistry, 2012, 43: 83-91. doi: 10.1016/j.orggeochem.2011.10.008 [31] MEI M, BISSADA K K, MALLOY T B, et al. Improved method for simultaneous determination of saturated and aromatic biomar-kers, organosulfur compounds and diamondoids in crude oils by GC-MS/MS[J]. Organic Geochemistry, 2018, 116: 35-50. doi: 10.1016/j.orggeochem.2017.09.010 [32] 马安来, 金之钧, 朱翠山. 塔里木盆地顺南1井原油硫代金刚烷系列的检出及意义[J]. 石油学报, 2018, 39(1): 42-53. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201801004.htmMA Anlai, JIN Zhijun, ZHU Cuishan. Detection and research significance of thiadiamondoids from crude oil in well Shunnan 1, Tarim Basin[J]. Acta Petrolei Sinica, 2018, 39(1): 42-53. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201801004.htm [33] 姜乃煌, 朱光有, 张水昌, 等. 塔里木盆地塔中83井原油中检测出2-硫代金刚烷及其地质意义[J]. 科学通报, 2007, 52(24): 2871-2875. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200724010.htmJIANG Naihuang, ZHU Guangyou, ZHANG Shuichang, et al. Detection of 2-thiaadamantanes in the oil from well TZ-83 in Tarim Basin and its geological implication[J]. Chinese Science Bulletin, 2008, 53(3): 396-401. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200724010.htm [34] HERNÁNDEZ F, PORTOLÉS T, PITARCH E, et al. Potential of gas chromatography coupled to triple quadrupole mass spectrometry for quantification and confirmation of organohalogen xenoestrogen compounds in human breast tissues[J]. Analytical Chemistry, 2005, 77(23): 7662-7672. doi: 10.1021/ac050874+ [35] QU Linjuan, HUI Zui, ZHU Jianhua, et al. Rapid determination of organophosphorous pesticides in leeks by gas chromatography-triple quadrupole mass spectrometry[J]. Food Chemistry, 2010, 122(1): 327-332. [36] WEI Zhibin, MOLDOWAN J M, ZHANG Shuichang, et al. Diamondoid hydrocarbons as a molecular proxy for thermal maturity and oil cracking: geochemical models from hydrous pyrolysis[J]. Organic Geochemistry, 2007, 38(2): 227-249. [37] FANG Chenchen, XIONG Yongqiang, LIANG Qianyong, et al. Variation in abundance and distribution of diamondoids during oil cracking[J]. Organic Geochemistry, 2012, 47: 1-8. [38] 房忱琛, 吴伟, 刘丹, 等. 煤系中金刚烷类化合物演化特征及应用[J]. 天然气地球科学, 2015, 26(1): 110-117. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201501014.htmFANG Chenchen, WU Wei, LIU Dan, et al. Evolution characte-ristics and application of diamondoids in coal measures[J]. Natural Gas Geoscience, 2015, 26(1): 110-117. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201501014.htm [39] FANG Chenchen, XIONG Yongqiang, LI Yun, et al. Generation and evolution of diamondoids in source rock[J]. Marine and Petroleum Geology, 2015, 67: 197-203. [40] WALTERS C C, WANG F C, QIAN Kuangnan, et al. Petroleum alteration by thermochemical sulfate reduction: a comprehensive molecular study of aromatic hydrocarbons and polar compounds[J]. Geochimica et Cosmochimica Acta, 2015, 153: 37-71. [41] GVIRTZMAN Z, SAID-AHMAD W, ELLIS G S, et al. Compound-specific sulfur isotope analysis of thiadiamondoids of oils from the Smackover Formation, USA[J]. Geochimica et Cosmochimica Acta, 2015, 167: 144-161. [42] CAI Chunfang, AMRANI A, WORDEN R H, et al. Sulfur isotopic compositions of individual organosulfur compounds and their genetic links in the Lower Paleozoic petroleum pools of the Tarim Basin, NW China[J]. Geochimica et Cosmochimica Acta, 2016, 182: 88-108. [43] 马安来, 金之钧, 朱翠山, 等. 塔里木盆地中深1C井原油高聚硫代金刚烷及金刚烷硫醇的检出及意义[J]. 中国科学(地球科学), 2018, 48(10): 1312-1323. https://www.cnki.com.cn/Article/CJFDTOTAL-JDXK201810004.htmMA Anlai, JIN Zhijun, ZHU Cuishan, et al. Detection and significance of higher thiadiamondoids and diamondoidthiols in oil from the Zhongshen 1C well of the Tarim Basin, NW China[J]. Science China(Earth Sciences), 2018, 61(10): 1440-1450. https://www.cnki.com.cn/Article/CJFDTOTAL-JDXK201810004.htm -





 下载:
下载:



 苏公网安备32021102000780号
苏公网安备32021102000780号