GC×GC-TOFMS analysis of ethanodiamondoids in Ordovician oil from well SN1, Tarim Basin
-
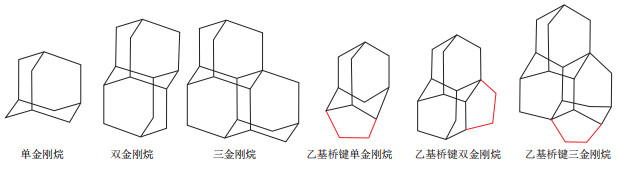
摘要: 运用全二维气相色谱—飞行时间质谱方法(GC×GC-TOFMS)从塔里木盆地顺托果勒地区顺南1井奥陶系原油中定量检测到81个1~3笼的乙基桥键金刚烷化合物,包括47个乙基桥键单金刚烷化合物,总含量为27 594.0 μg/g;32个乙基桥键双金刚烷化合物,总含量为4 415.1 μg/g;2个乙基桥键三金刚烷系列化合物,总含量为16.8 μg/g;建立了金刚烷—乙基桥键金刚烷的二维色谱保留指数图版。结果显示,金刚烷类和乙基桥键金刚烷类化合物保留时间位置具有如下关系:单金刚烷系列 < 乙基桥键单金刚烷系列 < 双金刚烷系列 < 乙基桥键双金刚烷系列 < 三金刚烷系列 < 乙基桥键三金刚烷系列 < 四金刚烷系列。作为石油中热稳定性最高的饱和烃类,乙基桥键金刚烷化合物的定量分析结果,有望为原油裂解和硫酸盐热化学还原反应(TSR)等油藏次生改造作用提供新的指标。
-
关键词:
- 乙基桥键金刚烷 /
- 全二维气相色谱—飞行时间质谱 /
- 奥陶系 /
- 顺南1井 /
- 塔里木盆地
Abstract: By 2D gas chromatography and time-of-flight mass spectrometry (GC×GC-TOFMS), 81 ethanodiamondoids with 1-3 cages were detected quantitatively in Ordovician crude oil from well SN1, Shuntuoguole area, Tarim Basin, including 47 ethanoadamantanes with total content of 27 594.0 μg/g, 32 ethanodiamantanes with total content of 4 415.1 μg/g, and 2 ethanotriamantanes with total content of 16.8 μg/g. The template of 2D chromatogram retention index of diamondoids-ethanodiamondoids was constructed. The results demonstrated that the position of retention time of diamondoids and ethanodiamondoids has the following relationship, namely, adamantanes < ethanoadamantanes < diamantanes < ethanodiamantanes < triadamantanes < ethanotriamantanes < tetradamantanes. Quantitative analytical result of ethanoadamantanes as saturated hydrocarbons with the highest thermal stability in crude oil, is expected to provide new indexes for secondary reservoir reconstruction, i.e. crude oil cracking and thermochemical sulfate reduction (TSR).-
Key words:
- ethanodiamondoids /
- GC×GC-TOFMS /
- Ordovician /
- well SN1 /
- Tarim Basin
-
表 1 塔里木盆地顺托果勒地区顺南1井原油中乙基桥键金刚烷定性和定量结果
Table 1. Qualitative and quantitative analysis results of ethanodiamondoids in crude oil from well SN1, Shuntuoguole area, Tarim Basin
序号 峰号 化合物名称 分子式 一维保留时间/s 二维保留时间/s 定量质量数 含量/(μg·g-1) 0 I.S.-1 D16-金刚烷 C10D16 1 796 1.41 152 332.9 1 EA-1 乙基桥键单金刚烷 C12H18 2 484 1.83 162 424.8 2 EA-2 1-甲基-乙基桥键单金刚烷 C13H20 2 524 1.65 161 737.4 3 EA-3 6-甲基-乙基桥键单金刚烷 C13H20 2 564 1.69 161 2 269.6 4 EA-4 2-甲基-乙基桥键单金刚烷 C13H20 2 596 1.77 161 833.8 5 EA-5 C1-乙基桥键单金刚烷 C13H20 2 620 1.74 161 77.5 6 EA-6 C1-乙基桥键单金刚烷 C13H20 2 676 1.83 161 314.7 7 EA-7 C1-乙基桥键单金刚烷 C13H20 2 700 1.88 161 87.4 8 EA-8 C2-乙基桥键单金刚烷 C14H22 2 884 1.77 161 402.8 9 EA-9 C2-乙基桥键单金刚烷 C14H22 2 892 1.81 161 477.2 10 EA-10 C2-乙基桥键单金刚烷 C14H22 2 596 1.51 175 2 196.1 11 EA-11 C2-乙基桥键单金刚烷 C14H22 2 636 1.55 175 1 713.5 12 EA-12 C2-乙基桥键单金刚烷 C14H22 2 660 1.56 175 358.9 13 EA-13 C2-乙基桥键单金刚烷 C14H22 2 668 1.64 175 930.3 14 EA-14 C2-乙基桥键单金刚烷 C14H22 2 692 1.57 175 668.5 15 EA-15 C2-乙基桥键单金刚烷 C14H22 2 700 1.59 175 1 139.1 16 EA-16 C2-乙基桥键单金刚烷 C14H22 2 700 1.7 175 153.7 17 EA-17 C2-乙基桥键单金刚烷 C14H22 2 716 1.64 175 733.0 18 EA-18 C2-乙基桥键单金刚烷 C14H22 2 740 1.66 175 1 794.8 19 EA-19 C2-乙基桥键单金刚烷 C14H22 2 756 1.66 175 715.7 20 EA-20 C2-乙基桥键单金刚烷 C14H22 2 772 1.72 175 452.5 21 EA-21 C2-乙基桥键单金刚烷 C14H22 2 780 1.79 175 375.5 22 EA-22 C2-乙基桥键单金刚烷 C14H22 2 796 1.73 175 706.9 23 EA-23 C2-乙基桥键单金刚烷 C14H22 2 812 1.76 175 505.8 24 EA-24 C2-乙基桥键单金刚烷 C14H22 2 836 1.79 175 150.9 25 EA-25 C2-乙基桥键单金刚烷 C14H22 2 852 1.82 175 357.1 26 EA-26 C2-乙基桥键单金刚烷 C14H22 2 908 1.91 175 156.3 27 EA-27 C3-乙基桥键单金刚烷 C15H24 2 684 1.45 189 493.2 28 EA-28 C3-乙基桥键单金刚烷 C15H24 2 716 1.46 189 345.8 29 EA-29 C3-乙基桥键单金刚烷 C15H24 2 724 1.41 189 1 261.2 30 EA-30 C3-乙基桥键单金刚烷 C15H24 2 732 1.5 189 141.2 31 EA-31 C3-乙基桥键单金刚烷 C15H24 2 764 1.47 189 931.0 32 EA-32 C3-乙基桥键单金刚烷 C15H24 2 804 1.51 189 388.9 33 EA-33 C3-乙基桥键单金刚烷 C15H24 2 828 1.61 189 188.8 34 EA-34 C3-乙基桥键单金刚烷 C15H24 2 844 1.56 189 763.4 35 EA-35 C3-乙基桥键单金刚烷 C15H24 2 852 1.59 189 1 069.3 36 EA-36 C3-乙基桥键单金刚烷 C15H24 2 860 1.59 189 277.5 37 EA-37 C3-乙基桥键单金刚烷 C15H24 2 868 1.56 189 457.6 38 EA-38 C3-乙基桥键单金刚烷 C15H24 2 884 1.65 189 271.9 39 EA-39 C4-乙基桥键单金刚烷 C16H26 2 740 1.26 203 211.5 40 EA-40 C4-乙基桥键单金刚烷 C16H26 2 764 1.29 203 634.2 41 EA-41 C4-乙基桥键单金刚烷 C16H26 2 828 1.43 203 51.2 42 EA-42 C4-乙基桥键单金刚烷 C16H26 2 852 1.46 203 439.4 43 EA-43 C4-乙基桥键单金刚烷 C16H26 2 860 1.47 203 194.8 44 EA-44 C4-乙基桥键单金刚烷 C16H26 2 892 1.44 203 222.7 45 EA-45 C4-乙基桥键单金刚烷 C16H26 2 964 1.52 203 427.8 46 EA-46 C5-乙基桥键单金刚烷 C17H28 2 860 1.17 217 75.9 47 EA-47 C5-乙基桥键单金刚烷 C17H28 2 916 1.2 217 13.2 48 ED-1 乙基桥键双金刚烷 C16H22 3 452 2.53 214 260.5 49 ED-2 乙基桥键双金刚烷 C16H22 3 540 2.62 214 52.6 50 ED-3 C1-乙基桥键双金刚烷 C17H24 3 468 2.27 213 103.9 51 ED-4 C1-乙基桥键双金刚烷 C17H24 3 476 2.25 213 197.4 52 ED-5 C1-乙基桥键双金刚烷 C17H24 3 500 2.32 213 322.6 53 ED-6 C1-乙基桥键双金刚烷 C17H24 3 532 2.41 213 129.3 54 ED-7 C1-乙基桥键双金刚烷 C17H24 3 556 2.45 213 115.1 55 ED-8 C1-乙基桥键双金刚烷 C17H24 3 564 2.32 213 85.9 56 ED-9 C1-乙基桥键双金刚烷 C17H24 3 604 2.49 213 41.8 57 ED-10 C1-乙基桥键双金刚烷 C17H24 3 620 2.6 213 72.5 58 ED-11 C1-乙基桥键双金刚烷 C17H24 3 636 2.57 213 33.2 59 ED-12 C1-乙基桥键双金刚烷 C17H24 3 652 2.57 213 101.8 60 ED-13 C1-乙基桥键双金刚烷 C17H24 3 676 2.54 213 29.5 61 ED-14 C1-乙基桥键双金刚烷 C17H24 3 684 2.65 213 53.1 62 ED-15 C1-乙基桥键双金刚烷 C17H24 3 692 2.6 213 27.1 63 ED-16 C2-乙基桥键双金刚烷 C18H26 3 772 2.38 213 49.4 64 ED-17 C2-乙基桥键双金刚烷 C18H26 3 780 2.43 213 57.5 65 ED-18 C2-乙基桥键双金刚烷 C18H26 3 476 1.95 227 312.9 66 ED-19 C2-乙基桥键双金刚烷 C18H26 3 524 2.06 227 125.6 67 ED-20 C2-乙基桥键双金刚烷 C18H26 3 548 2.14 227 148.7 68 ED-21 C2-乙基桥键双金刚烷 C18H26 3 564 2.19 227 165.8 69 ED-22 C2-乙基桥键双金刚烷 C18H26 3 596 2.21 227 111.2 70 ED-23 C2-乙基桥键双金刚烷 C18H26 3 628 2.21 227 97.2 71 ED-24 C2-乙基桥键双金刚烷 C18H26 3 652 2.26 227 303.7 72 ED-25 C2-乙基桥键双金刚烷 C18H26 3 700 2.38 227 149.4 73 ED-26 C2-乙基桥键双金刚烷 C18H26 3 724 2.45 227 119.5 74 ED-27 C2-乙基桥键双金刚烷 C18H26 3 756 2.41 227 128.5 75 ED-28 C2-乙基桥键双金刚烷 C18H26 3 788 2.51 227 311.7 76 ED-29 C3-乙基桥键双金刚烷 C19H28 3 492 1.75 241 272.4 77 ED-30 C3-乙基桥键双金刚烷 C19H28 3 572 1.88 241 59.8 78 ED-31 C3-乙基桥键双金刚烷 C19H28 3 748 2.21 241 249.4 79 ED-32 C3-乙基桥键双金刚烷 C19H28 3 796 2.21 241 126.3 80 ET-1 乙基桥键三金刚烷 C20H26 4 276 3.3 266 11.4 81 ET-2 C1-乙基桥键三金刚烷 C21H28 4 348 3.15 265 5.5 -
[1] STAUSS S, TERASHIMA K. Diamondoids: synthesis, properties, and applications[M]. Singapore: Pan Stanford Publishing, 2017. [2] 马安来, 金之钧, 晏继发, 等. 煤中金刚烷的分布与演化[J]. 石油学报, 2020, 41(7): 796-808. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB202007004.htmMA Anlai, JIN Zhijun, YAN Jifa, et al. Distribution and evolution of diamondoids in coals[J]. Acta Petrolei Sinica, 2020, 41(7): 796-808. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB202007004.htm [3] 马安来, 金之钧, 朱翠山, 等. 塔河油田原油中金刚烷化合物绝对定量分析[J]. 石油学报, 2009, 30(2): 214-218. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB200902010.htmMA Anlai, JIN Zhijun, ZHU Cuishan, et al. Quantitative analysis on absolute concentration of diamondoids in oils from Tahe Oilfield[J]. Acta Petrolei Sinica, 2009, 30(2): 214-218. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB200902010.htm [4] 房忱琛, 翟佳, 胡国艺, 等. 凝析油中金刚烷类和硫代金刚烷类化合物同步检测方法及地质意义: 以塔里木盆地塔中地区凝析油为例[J]. 石油实验地质, 2021, 43(5): 906-914. doi: 10.11781/sysydz202105906FANG Chenchen, ZHAI Jia, HU Guoyi, et al. A simultaneous determination method for diamondoids and thiadiamondoids in condensate oil and its geological significance: taking condensate oil from central Tarim Basin as an example[J]. Petroleum Geo-logy & Experiment, 2021, 43(5): 906-914. doi: 10.11781/sysydz202105906 [5] 杨茜, 包建平, 倪春华, 等. 蒸发分馏作用对原油中金刚烷类化合物分布与组成的影响: 以塔里木盆地库车坳陷羊塔克构造上的原油为例[J]. 石油实验地质, 2022, 44(2): 295-305. doi: 10.11781/sysydz202202295YANG Xi, BAO Jianping, NI Chunhua, et al. Effect of evaporative fractionation on the distribution and composition of diamondoids in crude oils: a case study of crude oils from Yangtake structure, Kuqa Depression, Tarim Basim[J]. Petroleum Geology & Experiment, 2022, 44(2): 295-305. doi: 10.11781/sysydz202202295 [6] SCHOELL M, CARLSON R M K. Diamondoids and oil are not forever[J]. Nature, 1999, 399(6731): 15-16. doi: 10.1038/19847 [7] 马安来. 金刚烷类化合物在有机地球化学中的应用进展[J]. 天然气地球科学, 2016, 27(5): 851-860. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201605012.htmMA Anlai. New advancement in application of diamondoids on organic geochemistry[J]. Natural Gas Geoscience, 2016, 27(5): 851-860. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201605012.htm [8] DAHL J E, LIU S G, CARLSON R M K. Isolation and structure of higher diamondoids, nanometer-sized diamond molecules[J]. Science, 2002, 299(5603): 96-99. http://www.econanotechnology.intermag.info/dahl-03.pdf [9] BURNS W, MCKERVEY M A, MITCHELL T R B, et al. A new approach to the construction of diamondoid hydrocarbons. Synthesis of anti-tetramantane[J]. Journal of the American Chemical Society, 1978, 100(3): 906-911. doi: 10.1021/ja00471a041 [10] FORT R C, VON R SCHLEYER P. Adamantane: consequences of the diamondoid structure[J]. Chemical Reviews, 1964, 64(3): 277-300. doi: 10.1021/cr60229a004 [11] MCKERVEY M A. Synthetic approaches to large diamondoid hydrocarbons[J]. Tetrahedron, 1980, 36(8): 971-992. doi: 10.1016/0040-4020(80)80050-0 [12] VON RAGUE SCHLEYER P, OSAWA E, DREW M G B. Nonacyclo[11.7.1.12, 18. 03, 16. 04, 13. 05, 10. 06, 14. 07, 11. 015, 20] docosane, a bastard tetramantane[J]. Journal of the American Che-mical Society, 1968, 90(18): 5034-5036. doi: 10.1021/ja01020a053 [13] GORDADZE G N, GIRUTS M V. Synthesis of adamantane and diamantane hydrocarbons by high-temperature cracking of higher n-alkanes[J]. Petroleum Chemistry, 2008, 48(6): 414-419. doi: 10.1134/S0965544108060029 [14] DAHL J E P, MOLDOWAN J M, WEI Zhibin, et al. Synthesis of higher diamondoids and implications for their formation in petroleum[J]. Angewandte Chemie, 2010, 122(51): 10077-10081. doi: 10.1002/ange.201004276 [15] CUPAS C A, HODAKOWSKI L. Iceane[J]. Journal of the AmericanChemical Society, 1974, 96(14): 4668-4669. [16] FARCASIU D, WISKOTT E, OSAWA E, et al. Ethanoadamantane. Most stable C12H18 isomer[J]. Journal of the American Chemical Society, 1974, 96(14): 4669-4671. doi: 10.1021/ja00821a051 [17] OSAWA E, FURUSAKI A, MATSUMOTO T, et al. Heptacyclo[8.6.0.02, 8. 03, 13. 04, 11. 05, 9. 012, 16] hexadecane a bisethano-bisnordiamantane by rearrangement[J]. Tetrahedron Letters, 1976, 17(28): 2463-2466. doi: 10.1016/0040-4039(76)90020-4 [18] RAO S T, SUNDARALINGAM M, OSAWA E, et al. Hexacyclo[10, 3, 1, 02, 10, , 03, 7, 06, 15, 09, 14] hexadecane; an ethanocongressane[J]. Journal of the Chemical Society D: Chemical Communications, 1970(14): 861-862. doi: 10.1039/c29700000861 [19] ZHU Guangyou, WANG Meng, ZHANG Ying, et al. Higher ethanodiamondoids in petroleum[J]. Energy & Fuels, 2018, 32(4): 4996-5000. http://www.onacademic.com/detail/journal_1000040213426510_8751.html [20] HÁLA S, LANDA S, HANUŠ V. Islolation of tetracyclo[6.3.1.02, 6. 05, 10] dodecane and pentacyclo[7.3.1.1. 4, 12. 02, 7. 06, 11] tetradecane (diamantane) from petroleum[J]. Angewandte Chemie International Edition, 1966, 5(12): 1045-1046. doi: 10.1002/anie.196610451 [21] BINGHAM R C, SCHLEYER P V R. Recent developments in the chemistry of adamantane and related polycyclic hydrocarbons[M]//BINGHAM R C, SCHLEYER P R V. Chemistry of Adamantanes. Berlin: Springer, 1971: 1-102. [22] SCARLETT A G, SPAAK G, MOHAMED S, et al. Comparison of tri-, tetra- and pentacyclic caged hydrocarbons in Australian crude oils and condensates[J]. Organic Geochemistry, 2019, 127: 115-123. http://www.sciencedirect.com/science/article/pii/S014663801830264X [23] ZHU Guangyou, LI Jingfei, WANG Meng, et al. Formation and distribution of ethanodiamondoids in deeply buried marine oil from the Tarim Basin, China[J]. Organic Geochemistry, 2021, 162: 104327. http://www.sciencedirect.com/science/article/pii/S0146638021001480 [24] 马安来, 林会喜, 云露, 等. 塔里木盆地顺托果勒地区奥陶系原油中乙基桥键金刚烷系列的检出及意义[J]. 石油学报, 2022, 43(6): 788-803. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB202206004.htmMA Anlai, LIN Huixi, YUN Lu, et al. Detection of ethanodiamondoids in the Ordovician crude oil from Shuntuoguole area in Tarim Basin and its significance[J]. Acta Petrolei Sinica, 2022, 43(4): 788-803. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB202206004.htm [25] 漆立新. 塔里木盆地顺北超深断溶体油藏特征与启示[J]. 中国石油勘探, 2020, 25(1): 102-111. https://www.cnki.com.cn/Article/CJFDTOTAL-KTSY202001010.htmQI Lixin. Characteristics and inspiration of ultra-deep fault-karst reservoir in the Shunbei area of the Tarim Basin[J]. China Petroleum Exploration, 2020, 25(1): 102-111. https://www.cnki.com.cn/Article/CJFDTOTAL-KTSY202001010.htm [26] 云露. 顺北地区奥陶系超深断溶体油气成藏条件[J]. 新疆石油地质, 2021, 42(2): 136-142. https://www.cnki.com.cn/Article/CJFDTOTAL-XJSD202102002.htmYUN Lu. Hydrocarbon accumulation of ultra-deep Ordovician fault-karst reservoirs in Shunbei area[J]. Xinjiang Petroleum Geology, 2021, 42(2): 136-142. https://www.cnki.com.cn/Article/CJFDTOTAL-XJSD202102002.htm [27] 吴鲜, 李丹, 朱秀香, 等. 塔里木盆地顺北油气田地温场对奥陶系超深层油气的影响: 以顺北5号走滑断裂带为例[J]. 石油实验地质, 2022, 44(3): 402-412. doi: 10.11781/sysydz202203402WU Xian, LI Dan, ZHU Xiuxiang, et al. Influence of geothermal field on ultra-deep Ordovician oil and gas in Shunbei field, Tarim Basin: a case study of Shunbei No. 5 strike-slip fault[J]. Petroleum Geology & Experiment, , 2022, 44(3): 402-412. doi: 10.11781/sysydz202203402 [28] 马安来, 何治亮, 云露, 等. 塔里木盆地顺北地区奥陶系超深层天然气地球化学特征及成因[J]. 天然气地球科学, 2021, 32(7): 1047-1060. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX202107011.htmMA Anlai, HE Zhiliang, YUN Lu, et al. The geochemical characteristics and origin of Ordovician ultra-deep natural gas in the North Shuntuoguole area, Tarim Basin, NW China[J]. Natural Gas Geoscience, 2021, 32(7): 1047-1060. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX202107011.htm [29] 庄新兵, 顾忆, 邵志兵, 等. 塔里木盆地地温场对油气成藏过程的控制作用: 以古城墟隆起为例[J]. 石油学报, 2017, 38(5): 502-511. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201705003.htmZHUANG Xinbing, GU Yi, SHAO Zhibing, et al. Control effectof geothermal field on hydrocarbon accumulation process in Tarim Basin: a case study of Guchengxu uplift[J]. Acta Petrolei Sinica, 2017, 38(5): 502-511. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201705003.htm [30] 马安来, 金之钧, 朱翠山. 等. 塔里木盆地顺南1井原油硫代金刚烷系列的检出及意义[J]. 石油学报, 2018, 39(1): 42-53. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201801004.htmMA Anlai, JIN Zhijun, ZHU Cuishan, et al. Detection and research significance of thiadiamondoids from crude oil in well Shunnan 1, Tarim Basin[J]. Acta Petrolei Sinica, 2018, 39(1): 42-53. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201801004.htm [31] 陈红汉, 鲁子野, 曹自成, 等. 塔里木盆地塔中地区北坡奥陶系热液蚀变作用[J]. 石油学报, 2016, 37(1): 43-63. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201601005.htmCHEN Honghan, LU Ziye, CAO Zicheng, et al. Hydrothermal alteration of Ordovician reservoir in northeastern slope of Tazhong Uplift, Tarim Basin[J]. Acta Petrolei Sinica, 2016, 37(1): 43-63. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201601005.htm [32] 沙旭光, 马庆佑, 吕海涛, 等. 塔里木盆地古城墟隆起奥陶系油气成藏特征及主控因素[J]. 海相油气地质, 2014, 19(2): 15-22. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYQ201402006.htmSHA Xuguang, MA Qingyou, LV Haitao, et al. Hydrocarbon accumulation and main controlling factors of Ordovician reservoir in Guchengxu uplift, Tarim Basin[J]. Marine Origin Petroleum Geo-logy, 2014, 19(2): 15-22. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYQ201402006.htm [33] OSAWA E, ENGLER E M, GODLESKI S A, et al. Application of force field calculations to organic chemistry. 10. Bridgehead reactivitiesof ethanoadamantane. Bromination and solvolysis of bromides[J]. The Journal of Organic Chemistry, 1980, 45(6): 984-991. doi: 10.1021/jo01294a014 [34] ÇAGIN T, CHE Jianwei, GARDOS M N, et al. Simulation and experiments on friction and wear of diamond: a material for MEMS and NEMS application[J]. Nanotechnology, 1999, 10(3): 278-284. http://www.wag.caltech.edu/home-pages/tahir/PDF/nems.pdf [35] PETERS K E, WALTERS C C, MOLDOWAN J M. The biomarkerguide volume 2: biomarkers and isotopes in petroleum exploration and earth history[M]. 2nd ed. Cambridge: Cambridge University Press, 2005. [36] 马安来, 金之钧, 李慧莉, 等. 塔里木盆地顺北地区奥陶系超深层油藏蚀变作用及保存[J]. 地球科学, 2020, 45(5): 1737-1753. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX202005017.htmMA Anlai, JIN Zhijun, Ll Huili, et al. Secondary alteration and preservation of ultra-deep Ordovician oil reservoirs of north Shuntuoguole area of Tarim Basin, NW China[J]. Earth Science, 2020, 45(5): 1737-1753. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX202005017.htm [37] DAHL J E, MOLDOWAN J M, PETERS K E, et al. Diamondoid hydrocarbons as indicators of natural oil cracking[J]. Nature, 1999, 399(6731): 54-57. http://www.nature.com/nature/journal/v399/n6731/pdf/399054a0.pdf -





 下载:
下载:







 苏公网安备32021102000780号
苏公网安备32021102000780号